We are passionate about our work. We support global clean energy transition by novel PV technology development.
We are passionate about our work and inspired by our team
Our process
PlasmaSolaris is a joint-venture between the high-profile scientific centers experts, entrepreneurs and investors. It involves research teams across European Union that work together for commercialization of the new generation of nano-plasmonic enhanced Solar Cells technologies and seek support of the EC funding (H2020).

1. Technological Research
The first phase of our process cycle is applied research that involves theory, modeling and experimental science.
2. Devices Prototyping and Testing
Further on we proceed to devices design and prototyping. The prototypes are then tested & verified technologically.
3. Technology Fine-Tuning
Upon outcomes of the prototypes functioning we employ a feed-back loop to optimize our technology and designs.
4. Devices Deployment
After the optimizations we are deploying the new devices commercially and provide our technological services.
5. Reiteration of the Cycle
We reiterate the process starting from research again to scientifically develop next generations of our technology.
Innovative Solar Cells PV technologies
Solar Cells research is a very dynamic field. It has many branches with the main division between a cheap but low efficient single-junction solar cells (either solid structures or advantagoues thin films) and expensive but highly efficient multi-junction cells (with architecture of many PV active layers). With nano-plasmonic enhancement we are working to provide simple single-juntrion solutions similar efficiencies which are attained in expensive devices.
- Multicrystalline Si Cells
Perovskite Solar Cells
Towards efficient & stable thin-film nanoplasmonic Solar Cells
Based on perovskite technology in which we identified a new channel of plasmons mediated efficiency enhancement
Research
Prototyping
Fine-tuning
Commercialization
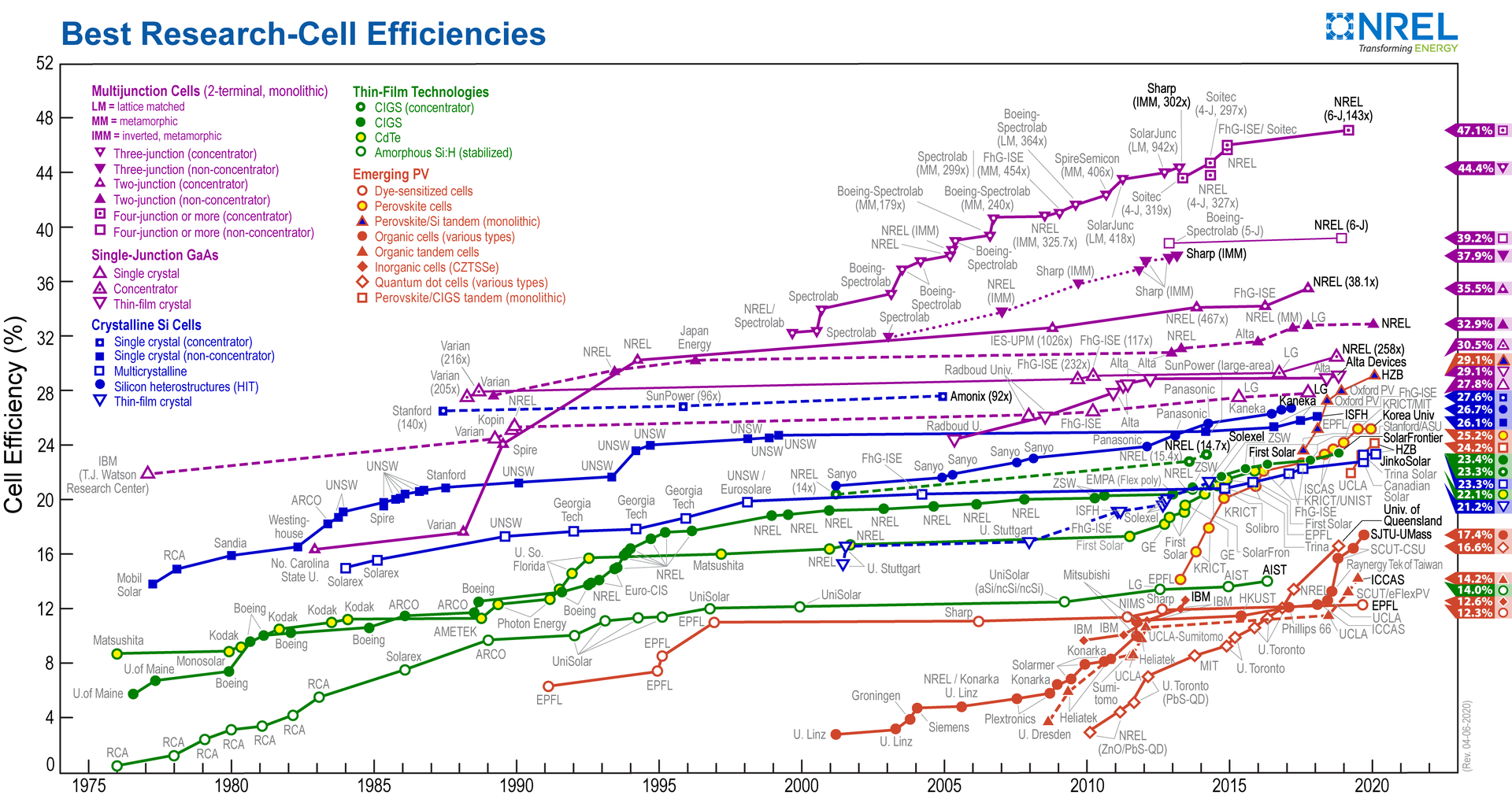
What is the current state of the art in Solar Cells?
A plasmonic-enhanced solar cell, commonly referred to simply as plasmonic solar cell, is a type of solar cell (including thin-film, crystalline silicon, amorphous silicon, and other types of cells) that converts light into electricity with the assistance of plasmons, but where the photovoltaic effect occurs in another material. A direct plasmonic solar cell is a solar cell that converts light into electricity using plasmons as the active, photovoltaic material. The thickness varies from that of traditional silicon PV, to less than 2 μm thick and theoretically could be as thin as 100 nm. They can use substrates which are cheaper than silicon, such as glass, plastic or steel. One of the challenges for thin film solar cells is that they do not absorb as much light as thicker solar cells made with materials with the same absorption coefficient. Methods for light trapping are important for thin film solar cells. Plasmonic-enhanced cells improve absorption by scattering light using metal nano-particles excited at their surface plasmon resonance, but more importantly due to involved quantum effects. Interestingly, plasmonic core-shell nanoparticles located in the front of the thin film solar cells can aid weak absorption of Si solar cells in the near-infrared region—the fraction of light scattered into the substrate and the maximum optical path length enhancement can be as high as 0.999 and 3133. Incoming light at the plasmon resonance frequency induces electron oscillations at the surface of the nanoparticles. The oscillation electrons can then be captured by a conductive layer producing an electrical current. The voltage produced is dependent on the bandgap of the conductive layer and the potential of the electrolyte in contact with the nanoparticles.
The Shockley–Queisser limit (also known as the detailed balance limit, Shockley Queisser Efficiency Limit or SQ Limit, or in physical terms the radiative efficiency limit) refers to the maximum theoretical efficiency of a solar cell using a single p-n junction to collect power from the cell where the only loss mechanism is radiative recombination in the solar cell. It was first calculated by William Shockley and Hans-Joachim Queisser at Shockley Semiconductor in 1961, giving a maximum efficiency of 30% at 1.1 eV. This first calculation used the 6000K black-body spectrum as an approximation to the solar spectrum. Subsequent calculations have used measured global solar spectra (AM1.5G) and included a back surface mirror which increases the maximum efficiency to 33.7% for a solar cell with a bandgap of 1.34 eV. The limit is one of the most fundamental to solar energy production with photovoltaic cells, and is considered to be one of the most important contributions in the field. The limit is that the maximum solar conversion efficiency is around 33.7% for a single p-n junction photovoltaic cell, assuming typical sunlight conditions (unconcentrated, AM 1.5 solar spectrum), and subject to other caveats and assumptions. This maximum occurs at a band gap of 1.34 eV. That is, of all the power contained in sunlight (about 1000 W/m²) falling on an ideal solar cell, only 33.7% of that could ever be turned into electricity (337 W/m²). The most popular solar cell material, silicon, has a less favorable band gap of 1.1 eV, resulting in a maximum efficiency of about 32%. Modern commercial mono-crystalline solar cells produce about 24% conversion efficiency, the losses due largely to practical concerns like reflection off the front of the cell and light blockage from the thin wires on the cell surface. The Shockley–Queisser limit only applies to conventional solar cells with a single p-n junction; tandem solar cells with multiple layers can (and do) outperform this limit, and so can solar thermal and certain other solar energy systems. In the extreme limit, for a tandem solar cell with an infinite number of layers, the corresponding limit is 86.8% using concentrated sunlight.
Gallium arsenide (GaAs) is an important semiconductor material for high-cost, high-efficiency solar cells and is used for single-crystalline thin film solar cells and for multi-junction solar cells. The first known operational use of GaAs solar cells in space was for the Soviet Venera 3 mission, launched in 1965. The GaAs solar cells, manufactured by Kvant, were chosen because of their higher performance in high temperature environments. GaAs cells were then used for the Lunokhod rovers for the same reason. In 1970, the GaAs heterostructure solar cells were developed by the team led by Zhores Alferov in the USSR, achieving much higher efficiencies. In the early 1980s, the efficiency of the best GaAs solar cells surpassed that of conventional, crystalline silicon-based solar cells. In the 1990s, GaAs solar cells took over from silicon as the cell type most commonly used for photovoltaic arrays for satellite applications. Later, dual- and triple-junction solar cells based on GaAs with germanium and indium gallium phosphide layers were developed as the basis of a triple-junction solar cell, which held a record efficiency of over 32% and can operate also with light as concentrated as 2,000 suns. This kind of solar cell powered the Mars Exploration Rovers Spirit and Opportunity, which explored Mars’ surface. Also many solar cars utilize GaAs in solar arrays. GaAs-based devices hold the world record for the highest-efficiency single-junction solar cell at 29.1 % (as of 2019). This high efficiency is attributed to the extreme high quality GaAs epitaxial growth, surface passivation by the AlGaAs, and the promotion of photon recycling by the thin film design. Complex designs of AlxGa1−xAs-GaAs devices using quantum wells can be sensitive to infrared radiation (QWIP). Beyond solar energy GaAs diodes can be used for the detection of X-rays.
Crystalline silicon (c-Si) is the crystalline forms of silicon, either polycrystalline silicon (poly-Si, consisting of small crystals), or monocrystalline silicon (mono-Si, a continuous crystal). Crystalline silicon is the dominant semiconducting material used in photovoltaic technology for the production of solar cells. These cells are assembled into most commonly adopted solar panels PV standard technology. In electronics, crystalline silicon is typically the monocrystalline form of silicon, and is used for producing microchips. This silicon contains much lower impurity levels than those required for solar cells. Production of semiconductor grade silicon involves a chemical purification to produce hyperpure polysilicon followed by a recrystallization process to grow monocrystalline silicon. The cylindrical boules are then cut into wafers for further processing. Solar cells made of crystalline silicon are often called conventional, traditional, or first generation solar cells, as they were developed in the 1950s and remained the most common type up to the present time. Because they are produced from 160–190 μm thick solar wafers—slices from bulks of solar grade silicon—they are sometimes called wafer-based solar cells. Solar cells made from c-Si are single-junction cells and are generally more efficient than their rival technologies, which are the second-generation thin-film solar cells, the most important being CdTe, CIGS, and amorphous silicon (a-Si). Amorphous silicon is an allotropic variant of silicon, and amorphous means “without shape” to describe its non-crystalline form.
Multi-junction (MJ) or tandem solar cells are solar cells with multiple p–n junctions made of different semiconductor materials. Each material’s p-n junction will produce electric current in response to different wavelengths of light. The use of multiple semiconducting materials allows the absorbance of a broader range of wavelengths, improving the cell’s sunlight to electrical energy conversion efficiency. Traditional single-junction cells have a maximum theoretical efficiency of 33.16%. Theoretically, an infinite number of junctions would have a limiting efficiency of 86.8% under highly concentrated sunlight. Currently, the best lab examples of traditional crystalline silicon (c-Si) solar cells have efficiencies between 20% and 25%, while lab examples of multi-junction cells have demonstrated performance over 46% under concentrated sunlight. Commercial examples of tandem cells are widely available at 30% under one-sun illumination, and improve to around 40% under concentrated sunlight. However, this efficiency is gained at the cost of increased complexity and manufacturing price. To date, their higher price and higher price-to-performance ratio have limited their use to special roles, notably in aerospace where their high power-to-weight ratio is desirable. In terrestrial applications, these solar cells are emerging in concentrator photovoltaics (CPV), with a growing number of installations around the world. Tandem fabrication techniques have been used to improve the performance of existing designs. In particular, the technique can be applied to lower cost thin-film solar cells using amorphous silicon, as opposed to conventional crystalline silicon, to produce a cell with about 10% efficiency that is lightweight and flexible. This approach has been used by several commercial vendors, but these products are currently limited to certain niche roles, like roofing materials.
A quantum dot solar cell (QDSC) is a solar cell design that uses quantum dots as the absorbing photovoltaic material. It attempts to replace bulk materials such as silicon, copper indium gallium selenide (CIGS) or cadmium telluride (CdTe). Quantum dots have bandgaps that are tunable across a wide range of energy levels by changing their size. In bulk materials, the bandgap is fixed by the choice of material(s). This property makes quantum dots attractive for multi-junction solar cells, where a variety of materials are used to improve efficiency by harvesting multiple portions of the solar spectrum. As of 2019, efficiency exceeds 16.5%. The Shockley-Queisser limit, which sets the maximum efficiency of a single-layer photovoltaic cell to be 33.7%, assumes that only one electron-hole pair (exciton) can be generated per incoming photon. Multiple exciton generation (MEG) is an exciton relaxation pathway which allows two or more excitons to be generated per incoming high energy photon. In traditional photovoltaics, this excess energy is lost to the bulk material as lattice vibrations (electron-phonon coupling). MEG occurs when this excess energy is transferred to excite additional electrons across the band gap, where they can contribute to the short-circuit current density. Within quantum dots, quantum confinement increases coulombic interactions which drives the MEG process. This phenomenon also decreases the rate of electron-phonon coupling, which is the dominant method of exciton relaxation in bulk semiconductors. The phonon bottleneck slows the rate of hot carrier cooling, which allows excitons to pursue other pathways of relaxation; this allows MEG to dominate in quantum dot solar cells. The rate of MEG can be optimized by tailoring quantum dot ligand chemistry, as well as by changing the quantum dot material and geometry. In 2004, Los Alamos National Laboratory reported spectroscopic evidence that several excitons could be efficiently generated upon absorption of a single, energetic photon in a quantum dot. Capturing them would catch more of the energy in sunlight. In this approach, known as “carrier multiplication” (CM) or “multiple exciton generation” (MEG), the quantum dot is tuned to release multiple electron-hole pairs at a lower energy instead of one pair at high energy. This increases efficiency through increased photocurrent. LANL’s dots were made from lead selenide. In 2010, the University of Wyoming demonstrated similar performance using DCCS cells. Lead-sulfur (PbS) dots demonstrated two-electron ejection when the incoming photons had about three times the bandgap energy. In 2005, NREL demonstrated MEG in quantum dots, producing three electrons per photon and a theoretical efficiency of 65%. In 2007, they achieved a similar result in silicon. Although quantum dot solar cells have yet to be commercially viable on the mass scale, several small commercial providers have begun marketing quantum dot photovoltaic products. Investors and financial analysts have identified quantum dot photovoltaics as a key future technology for the solar industry. Many heavy-metal quantum dot (lead/cadmium chalcogenides such as PbSe, CdSe) semiconductors can be cytotoxic and must be encapsulated in a stable polymer shell to prevent exposure. Non-toxic quantum dot materials such as AgBiS2 nanocrystals have been explored due to their safety and abundance; exploration with solar cells based with these materials have demonstrated comparable conversion efficiencies and short-circuit current densities. UbiQD’s CuInSe2-X quantum dot material is another example of a non-toxic semiconductor compound.
Concentrator photovoltaics (CPV, also known as concentration photovoltaics) is a photovoltaic technology that unlike conventional photovoltaic systems, uses lenses or curved mirrors to focus sunlight onto small solar cells (usually, highly efficient, multi-junction solar cells). In addition, CPV systems often use solar trackers and sometimes a cooling system to further increase their efficiency. Ongoing research and development is rapidly improving their competitiveness in the utility-scale segment and in areas of high insolation. Systems using high-concentration photovoltaics (HCPV) especially have the potential to become competitive in the near future. They possess the highest efficiency of all existing PV technologies, and a smaller photovoltaic array also reduces the balance of system costs. Currently, CPV is far less common than conventional PV systems and has only recently been made available to the residential market. In 2016, cumulative CPV installations reached 350 megawatts (MW), less than 0.2% of the global installed capacity of 230,000 MW. Commercial HCPV systems reached instantaneous (“spot”) efficiencies of up to 42% under standard test conditions (with concentration levels above 400) and the International Energy Agency sees potential to increase the efficiency of this technology to 50% by the mid-2020s. As of December 2014, the best lab cell efficiency for concentrator MJ-cells reached 46% (four or more junctions). Under outdoor, operating conditions, CPV module efficiencies have exceeded 33% (“one third of a sun”). System-level AC efficiencies are in the range of 25-28%. CPV installations are located in China, the United States, South Africa, Italy and Spain. HCPV directly competes with concentrated solar power (CSP) as both technologies are suited best for areas with high direct normal irradiance, which are also known as the Sun Belt region in the United States and the Golden Banana in Southern Europe. CPV and CSP are often confused with one another, despite being intrinsically different technologies from the start: CPV uses the PV effect to directly generate electricity from sunlight, while CSP – often called concentrated solar thermal – uses the heat from the sun’s radiation in order to make steam to drive a turbine, that then produces electricity using a generator.
A perovskite solar cell is a type of solar cell which includes a perovskite structured compound, most commonly a hybrid organic-inorganic lead or tin halide-based material, as the light-harvesting active layer. Perovskite materials, such as methylammonium lead halides and all-inorganic cesium lead halide, are cheap to produce and simple to manufacture. Solar cell efficiencies of devices using these materials have increased from 3.8% in 2009 to 25.2% in 2020 in single-junction architectures, and, in silicon-based tandem cells, to 29.1%, exceeding the maximum efficiency achieved in single-junction silicon solar cells. Perovskite solar cells are therefore currently the fastest-advancing solar technology. With the potential of achieving even higher efficiencies and very low production costs, perovskite solar cells have become commercially attractive.
A copper indium gallium selenide solar cell (or a CIGS cell) is a thin-film solar cell manufactured by depositing a thin layer of copper, indium, gallium and selenium on glass or plastic backing, along with electrodes on the front and back to collect current. Because the material has a high absorption coefficient and strongly absorbs sunlight, a much thinner film is required than of other semiconductor materials. CIGS is one of three mainstream thin-film PV technologies, the other two being cadmium telluride and amorphous silicon. Like these materials, CIGS layers are thin enough to be flexible, allowing them to be deposited on flexible substrates. However, as all of these technologies normally use high-temperature deposition techniques, the best performance normally comes from cells deposited on glass, even though advances in low-temperature deposition of CIGS cells have erased much of this performance difference. CIGS outperforms polysilicon at the cell level, however its module efficiency is still lower, due to a less mature upscaling. Thin-film market share is stagnated at around 15 percent, leaving the rest of the PV market to conventional solar cells made of crystalline silicon. In 2013, the market share of CIGS alone was about 2 percent and all thin-film technologies combined fell below 10 percent. CIGS cells continue being developed, as they promise to reach silicon-like efficiencies, while maintaining their low costs, as is typical for thin-film technology. Prominent manufacturers of CIGS photovoltaics were the now-bankrupt companies Nanosolar and Solyndra. Current market leader is the Japanese company Solar Frontier, with Global Solar and GSHK Solar also producing solar modules free of any heavy metals such as cadmium and/or lead.
Cadmium telluride (CdTe) photovoltaics describes a photovoltaic (PV) technology that is based on the use of cadmium telluride in a thin semiconductor layer designed to absorb and convert sunlight into electricity. Cadmium telluride PV is the only thin film technology with lower costs than conventional solar cells made of crystalline silicon in multi-kilowatt systems. On a lifecycle basis, CdTe PV has the smallest carbon footprint, lowest water use and shortest energy payback time of any current photo voltaic technology. CdTe’s energy payback time of less than a year allows for faster carbon reductions without short-term energy deficits. The toxicity of cadmium is an environmental concern mitigated by the recycling of CdTe modules at the end of their life time. Though there are still uncertainties regarding the recycling of CdTe modules and the public opinion is skeptical towards this technology. The usage of rare materials may also become a limiting factor to the industrial scalability of CdTe technology in the mid-term future. The abundance of tellurium—of which telluride is the anionic form—is comparable to that of platinum in the earth’s crust and contributes significantly to the module’s cost. CdTe photovoltaics are used in some of the world’s largest photovoltaic power stations, such as the Topaz Solar Farm. With a share of 5.1% of worldwide PV production, CdTe technology accounted for more than half of the thin film market in 2013. A prominent manufacturer of CdTe thin film technology is the company First Solar, based in Tempe, Arizona.
Amorphous silicon (a-Si) cells are based on the non-crystalline form of silicon. Used as semiconductor material for thin-film silicon solar cells, a-Si is deposited in thin films onto a variety of flexible substrates, such as glass, metal and plastic. Amorphous silicon cells generally feature low efficiency, but are one of the most environmentally friendly photovoltaic technologies, since they do not use any toxic heavy metals such as cadmium or lead. As a second-generation thin-film solar cell technology, amorphous silicon was once expected to become a major contributor in the fast-growing worldwide photovoltaic market, but has since lost its significance due to strong competition from conventional crystalline silicon cells and other thin-film technologies such as CdTe, CIGS and perovskite. Amorphous silicon differs from other allotropic variations, such as monocrystalline silicon—a single crystal, and polycrystalline silicon, that consists of small grains, also known as crystallites. Silicon is a fourfold coordinated atom that is normally tetrahedrally bonded to four neighboring silicon atoms. In crystalline silicon (c-Si) this tetrahedral structure continues over a large range, thus forming a well-ordered crystal lattice. In amorphous silicon this long range order is not present. Rather, the atoms form a continuous random network. Moreover, not all the atoms within amorphous silicon are fourfold coordinated. Due to the disordered nature of the material some atoms have a dangling bond. Physically, these dangling bonds represent defects in the continuous random network and may cause anomalous electrical behavior. The material can be passivated by hydrogen, which bonds to the dangling bonds and can reduce the dangling bond density by several orders of magnitude. Hydrogenated amorphous silicon (a-Si:H) has a sufficiently low amount of defects to be used for solar photovoltaic cells, particularly in the protocrystalline growth regime. However, hydrogenation is associated with light-induced degradation of the material, termed the Staebler–Wronski effect.
A dye-sensitized solar cell (DSSC, DSC, DYSC or Grätzel cell) is a low-cost solar cell belonging to the group of thin film solar cells. It is based on a semiconductor formed between a photo-sensitized anode and an electrolyte, a photoelectrochemical system. The modern version of a dye solar cell, also known as the Grätzel cell, was originally co-invented in 1988 by Brian O’Regan and Michael Grätzel at UC Berkeley and this work was later developed by the aforementioned scientists at the École Polytechnique Fédérale de Lausanne until the publication of the first high efficiency DSSC in 1991. Michael Grätzel has been awarded the 2010 Millennium Technology Prize for this invention. The DSSC has a number of attractive features. It is simple to make using conventional roll-printing techniques, is semi-flexible and semi-transparent which offers a variety of uses not applicable to glass-based systems, and most of the materials used are low-cost. In practice it has proven difficult to eliminate a number of expensive materials, notably platinum and ruthenium, and the liquid electrolyte presents a serious challenge to making a cell suitable for use in all weather. Although its conversion efficiency is less than the best thin-film cells, in theory its price/performance ratio should be good enough to allow them to compete with fossil fuel electrical generation by achieving grid parity. Commercial applications, which were held up due to chemical stability problems, are forecast in the European Union Photovoltaic Roadmap to significantly contribute to renewable electricity generation by 2020.
An organic solar cell (OSC) or plastic solar cell is a type of photovoltaic that uses organic electronics, a branch of electronics that deals with conductive organic polymers or small organic molecules, for light absorption and charge transport to produce electricity from sunlight by the photovoltaic effect. Most organic photovoltaic cells are polymer solar cells. The molecules used in organic solar cells are solution-processable at high throughput and are cheap, resulting in low production costs to fabricate a large volume. Combined with the flexibility of organic molecules, organic solar cells are potentially cost-effective for photovoltaic applications. Molecular engineering (e.g. changing the length and functional group of polymers) can change the band gap, allowing for electronic tunability. The optical absorption coefficient of organic molecules is high, so a large amount of light can be absorbed with a small amount of materials, usually on the order of hundreds of nanometers. The main disadvantages associated with organic photovoltaic cells are low efficiency, low stability and low strength compared to inorganic photovoltaic cells such as silicon solar cells. Compared to silicon-based devices, polymer solar cells are lightweight (which is important for small autonomous sensors), potentially disposable and inexpensive to fabricate (sometimes using printed electronics), flexible, customizable on the molecular level and potentially have less adverse environmental impact. Polymer solar cells also have the potential to exhibit transparency, suggesting applications in windows, walls, flexible electronics, etc. The disadvantages of polymer solar cells are also serious: they offer about 1/3 of the efficiency of hard materials, and experience substantial photochemical degradation. Polymer solar cells inefficiency and stability problems, combined with their promise of low costs and increased efficiency made them a popular field in solar cell research. As of 2015, polymer solar cells were able to achieve over 10% efficiency via a tandem structure. In 2018, a record breaking efficiency for organic photovoltaics of 17.3% was reached via tandem structure.
Copper zinc tin sulfide (CZTS) is a quaternary semiconducting compound which has received increasing interest since the late 2000s for applications in thin film solar cells. The class of related materials includes other I2-II-IV-VI4 such as copper zinc tin selenide (CZTSe) and the sulfur-selenium alloy CZTSSe. CZTS offers favorable optical and electronic properties similar to CIGS (copper indium gallium selenide), making it well suited for use as a thin-film solar cell absorber layer, but unlike CIGS (or other thin films such as CdTe), CZTS is composed of only abundant and non-toxic elements. Concerns with the price and availability of indium in CIGS and tellurium in CdTe, as well as toxicity of cadmium have been a large motivator to search for alternative thin film solar cell materials. The power conversion efficiency of CZTS is still considerably lower than CIGS and CdTe, with laboratory cell records of 11.0 % for CZTS and 12.6 % for CZTSSe as of 2019.
Familiarize yourself with the nanoplasmonic photovoltaics
Would like to get in touch? Please contact us

BROUGHT BY